Page 13 - EASL Recommendations on Treatment of Hepatitis C 2015
P. 13
JOURNAL OF HEPATOLOGY
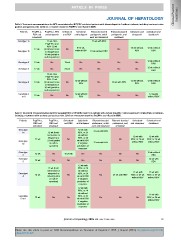
Table 5. Treatment recommendations for HCV-monoinfected or HCV/HIV coinfected patients with chronic hepatitis C without cirrhosis, including treatment-naïve
patients and patients who failed on a treatment based on PegIFN-a and ribavirin (RBV).
Patients PegIFN-α, PegIFN-α, RBV Sofosbuvir Sofosbuvir Ritonavir-boosted Ritonavir-boosted Sofosbuvir and Sofosbuvir and
RBV and and simeprevir and RBV and ledipasvir paritaprevir, ombit- paritaprevir, and simeprevir daclatasvir
sofosbuvir
asvir and dasabuvir ombitasvir 12 wk without 12 wk without
RBV RBV
Genotype 1a 12 wk 12 wk, then No 12 wk with RBV No
Genotype 1b PegIFN-α and No 12 wk without
8-12 wk, No No RBV
RBV 12 wk without RBV 12 wk without RBV No
(treatment-naïve 12 wk without 12 wk without
or relapsers) or 12 wk with RBV RBV RBV
36 wk (partial or
null responders) No No 12 wk without
RBV
Genotype 2 12 wk No 12 wk No No
12 weeks
Genotype 3 12 wk No 24 wk No No without RBV
12 wk No No
Genotype 4 12 wk 12 wk, then No 12 wk without No
PegIFN-α and RBV
Genotype 5
or 6 RBV 12 wk 12 wk without
(treatment-naïve RBV
or relapsers) or
36 wk (partial or
null responders)
No
Table 6. Treatment recommendations for HCV-monoinfected or HCV/HIV coinfected patients with chronic hepatitis C with compensated (Child-Pugh A) cirrhosis,
including treatment-naïve patients and patients who failed on a treatment based on PegIFN-a and ribavirin (RBV).
Patients PegIFN-α, PegIFN-α, Sofosbuvir Sofosbuvir Ritonavir-boosted Ritonavir-boosted Sofosbuvir Sofosbuvir and
RBV and RBV and and RBV
Genotype sofosbuvir simeprevir and ledipasvir paritaprevir, ombit- paritaprevir, and and simeprevir daclatasvir
1a No
12 wk 12 wk (treat- 16-20 wk asvir and dasabuvir ombitasvir
Genotype ment-naïve or
1b 12 wk relapsers) or No 12 wk with 24 wk with RBV 12 wk with 12 wk with
12 wk 24 wk (partial No RBV, or 24 12 wk with RBV
Genotype wk without No RBV, or 24 wk RBV, or 24 wk
2 12 wk or null re- No RBV, or 24
sponders) wk with RBV without RBV without RBV
Genotype 12 wk if negative
3 No predictors of
response
Genotype No
4 No No 12 wk without
12 wk (treat- No No
Genotype ment-naïve or
5 or 6 relapsers) or RBV
24 wk (partial
No No No No 24 wk with
or null re- RBV
sponders)
12 wk with
No
RBV, or 24
wk without 12 wk with 12 wk with
RBV, or 24
wk with RBV No 24 wk with RBV RBV, or 24 wk RBV, or 24 wk
if negative without RBV without RBV
predictors of
response
12 wk with No 12 wk with
RBV, or 24 No No RBV, or 24 wk
wk without
RBV, or 24 without RBV
wk with RBV
if negative
predictors of
response
Journal of Hepatology 2015 vol. xxx j xxx–xxx 13
Please cite this article in press as: EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol (2015), http://dx.doi.org/10.1016/
j.jhep.2015.03.025
Table 5. Treatment recommendations for HCV-monoinfected or HCV/HIV coinfected patients with chronic hepatitis C without cirrhosis, including treatment-naïve
patients and patients who failed on a treatment based on PegIFN-a and ribavirin (RBV).
Patients PegIFN-α, PegIFN-α, RBV Sofosbuvir Sofosbuvir Ritonavir-boosted Ritonavir-boosted Sofosbuvir and Sofosbuvir and
RBV and and simeprevir and RBV and ledipasvir paritaprevir, ombit- paritaprevir, and simeprevir daclatasvir
sofosbuvir
asvir and dasabuvir ombitasvir 12 wk without 12 wk without
RBV RBV
Genotype 1a 12 wk 12 wk, then No 12 wk with RBV No
Genotype 1b PegIFN-α and No 12 wk without
8-12 wk, No No RBV
RBV 12 wk without RBV 12 wk without RBV No
(treatment-naïve 12 wk without 12 wk without
or relapsers) or 12 wk with RBV RBV RBV
36 wk (partial or
null responders) No No 12 wk without
RBV
Genotype 2 12 wk No 12 wk No No
12 weeks
Genotype 3 12 wk No 24 wk No No without RBV
12 wk No No
Genotype 4 12 wk 12 wk, then No 12 wk without No
PegIFN-α and RBV
Genotype 5
or 6 RBV 12 wk 12 wk without
(treatment-naïve RBV
or relapsers) or
36 wk (partial or
null responders)
No
Table 6. Treatment recommendations for HCV-monoinfected or HCV/HIV coinfected patients with chronic hepatitis C with compensated (Child-Pugh A) cirrhosis,
including treatment-naïve patients and patients who failed on a treatment based on PegIFN-a and ribavirin (RBV).
Patients PegIFN-α, PegIFN-α, Sofosbuvir Sofosbuvir Ritonavir-boosted Ritonavir-boosted Sofosbuvir Sofosbuvir and
RBV and RBV and and RBV
Genotype sofosbuvir simeprevir and ledipasvir paritaprevir, ombit- paritaprevir, and and simeprevir daclatasvir
1a No
12 wk 12 wk (treat- 16-20 wk asvir and dasabuvir ombitasvir
Genotype ment-naïve or
1b 12 wk relapsers) or No 12 wk with 24 wk with RBV 12 wk with 12 wk with
12 wk 24 wk (partial No RBV, or 24 12 wk with RBV
Genotype wk without No RBV, or 24 wk RBV, or 24 wk
2 12 wk or null re- No RBV, or 24
sponders) wk with RBV without RBV without RBV
Genotype 12 wk if negative
3 No predictors of
response
Genotype No
4 No No 12 wk without
12 wk (treat- No No
Genotype ment-naïve or
5 or 6 relapsers) or RBV
24 wk (partial
No No No No 24 wk with
or null re- RBV
sponders)
12 wk with
No
RBV, or 24
wk without 12 wk with 12 wk with
RBV, or 24
wk with RBV No 24 wk with RBV RBV, or 24 wk RBV, or 24 wk
if negative without RBV without RBV
predictors of
response
12 wk with No 12 wk with
RBV, or 24 No No RBV, or 24 wk
wk without
RBV, or 24 without RBV
wk with RBV
if negative
predictors of
response
Journal of Hepatology 2015 vol. xxx j xxx–xxx 13
Please cite this article in press as: EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol (2015), http://dx.doi.org/10.1016/
j.jhep.2015.03.025