Page 25 - EASL Recommendations on Treatment of Hepatitis C 2015 - Full version
P. 25
Clinical Practice Guidelines drugs, number of drugs used, use of ribavirin, treatment dura-
tion). Whether assessing the sequence of the target HCV genes
paritaprevir), an NS5A inhibitor (daclatasvir, ledipasvir, ombitas- (HCV resistance testing) prior to retreatment is helpful to make
vir) or a non-nucleoside inhibitor of HCV polymerase (dasabuvir) a decision remains unknown, as well as which therapeutic
who fail to achieve SVR select viruses with amino acid sub- decision should be made based on this result.
stitutions in the NS3 protease, NS5A and polymerase regions,
respectively, that confer drug resistance. Viruses resistant to pro- Intuitively, patients who failed on a DAA-containing regimen
tease inhibitors and, probably also but more slowly, viruses resis- should be retreated with an IFN-free combination including a
tant to non-nucleoside polymerase inhibitors progressively drug with a high barrier to resistance (currently, sofosbuvir), plus
decrease in proportion to become undetectable by means of one or two other drugs, ideally with no cross-resistance with the
population sequencing (direct sequence analysis) within a few drugs already administered. Based on results in difficult-to-cure
months to 2 years after treatment cessation. In contrast, viruses patient populations, retreatment should be for 12 weeks with
resistant to NS5A inhibitors are fit and remain dominant for ribavirin, or extended to 24 weeks with or without ribavirin (no
many years, perhaps forever, after they have been selected by a data available comparing these approaches).
regimen including an NS5A inhibitor [80–86].
Patients who failed on sofosbuvir alone or sofosbuvir plus
Currently, there is no data to firmly support retreatment
recommendations, which must be based on indirect evidence ribavirin or sofosbuvir plus PegIFN-a and ribavirin can be
(HCV genotype, known resistance profiles of the administered
retreated with a combination of sofosbuvir plus simeprevir
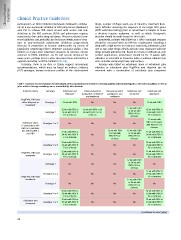
Table 7. Treatment recommendations for retreatment of HCV-monoinfected or HCV/HIV coinfected patients with chronic hepatitis C who failed to achieve an SVR on
prior antiviral therapy containing one or several DAA(s) RBV: ribavirin.
Failed treatment Genotype Sofosbuvir and Ritonavir-boosted Ritonavir-boosted Sofosbuvir and Sofosbuvir and
Genotype 1 ledipasvir paritaprevir, ombitasvir paritaprevir, and simeprevir daclatasvir
PegIFN-α, RBV and Genotype 1
either telaprevir or 12 wk with RBV and dasabuvir ombitasvir No 12 wk with RBV
boceprevir No No 12 wk with RBV 12 wk with RBV or
or 24 wk with 24 wk with RBV if
12 wk with RBV or 12 wk with RBV or 24 No RBV if F3 or
24 wk with RBV if wk with RBV if F3 or cirrhosis F3 or cirrhosis
F3 or cirrhosis cirrhosis No 12 weeks with
RBV or 24 weeks
Sofosbuvir alone, Genotype 2 or 3 No No No 12 wk with RBV with RBV if F3 or
in combination with Genotype 4 No or 24 wk with
RBV or in combina- 12 wk with RBV or No 12 wk with RBV RBV if F3 or cirrhosis
tion with PegIFN-α 24 wk with RBV if No or 24 wk with cirrhosis
No RBV if F3 or 12 wk with RBV or
and RBV F3 or cirrhosis cirrhosis No 24 wk with RBV if
No
PegIFN-α, RBV and Genotype 5 or 6 12 wk with RBV or No F3 or cirrhosis
simeprevir Genotype 1 or 4 24 wk with RBV if No
12 wk with RBV 12 wk with RBV or
F3 or cirrhosis No or 24 wk with 24 wk with RBV if
RBV if F3 or
12 wk with RBV or cirrhosis F3 or cirrhosis
24 wk with RBV if 12 wk with RBV or
No 24 wk with RBV if
F3 or cirrhosis
12 wk with RBV F3 or cirrhosis
Genotype 1 No or 24 wk with
RBV if F3 or No
PegIFN-α, RBV and Genotype 2 or 3 No No No cirrhosis
daclatasvir Genotype 4 No No No 12 wk with RBV or
No No No 24 wk with RBV if
Sofosbuvir and Genotype 5 or 6 12 wk with RBV or No No
simeprevir Genotype 1 or 4 24 wk with RBV if No F3 or cirrhosis
F3 or cirrhosis No
12 wk with RBV or 12 wk with RBV or
24 wk with RBV if 24 wk with RBV if
F3 or cirrhosis F3 or cirrhosis
12 wk with RBV or
24 wk with RBV if
F3 or cirrhosis
(continued on next page)
24
tion). Whether assessing the sequence of the target HCV genes
paritaprevir), an NS5A inhibitor (daclatasvir, ledipasvir, ombitas- (HCV resistance testing) prior to retreatment is helpful to make
vir) or a non-nucleoside inhibitor of HCV polymerase (dasabuvir) a decision remains unknown, as well as which therapeutic
who fail to achieve SVR select viruses with amino acid sub- decision should be made based on this result.
stitutions in the NS3 protease, NS5A and polymerase regions,
respectively, that confer drug resistance. Viruses resistant to pro- Intuitively, patients who failed on a DAA-containing regimen
tease inhibitors and, probably also but more slowly, viruses resis- should be retreated with an IFN-free combination including a
tant to non-nucleoside polymerase inhibitors progressively drug with a high barrier to resistance (currently, sofosbuvir), plus
decrease in proportion to become undetectable by means of one or two other drugs, ideally with no cross-resistance with the
population sequencing (direct sequence analysis) within a few drugs already administered. Based on results in difficult-to-cure
months to 2 years after treatment cessation. In contrast, viruses patient populations, retreatment should be for 12 weeks with
resistant to NS5A inhibitors are fit and remain dominant for ribavirin, or extended to 24 weeks with or without ribavirin (no
many years, perhaps forever, after they have been selected by a data available comparing these approaches).
regimen including an NS5A inhibitor [80–86].
Patients who failed on sofosbuvir alone or sofosbuvir plus
Currently, there is no data to firmly support retreatment
recommendations, which must be based on indirect evidence ribavirin or sofosbuvir plus PegIFN-a and ribavirin can be
(HCV genotype, known resistance profiles of the administered
retreated with a combination of sofosbuvir plus simeprevir
Table 7. Treatment recommendations for retreatment of HCV-monoinfected or HCV/HIV coinfected patients with chronic hepatitis C who failed to achieve an SVR on
prior antiviral therapy containing one or several DAA(s) RBV: ribavirin.
Failed treatment Genotype Sofosbuvir and Ritonavir-boosted Ritonavir-boosted Sofosbuvir and Sofosbuvir and
Genotype 1 ledipasvir paritaprevir, ombitasvir paritaprevir, and simeprevir daclatasvir
PegIFN-α, RBV and Genotype 1
either telaprevir or 12 wk with RBV and dasabuvir ombitasvir No 12 wk with RBV
boceprevir No No 12 wk with RBV 12 wk with RBV or
or 24 wk with 24 wk with RBV if
12 wk with RBV or 12 wk with RBV or 24 No RBV if F3 or
24 wk with RBV if wk with RBV if F3 or cirrhosis F3 or cirrhosis
F3 or cirrhosis cirrhosis No 12 weeks with
RBV or 24 weeks
Sofosbuvir alone, Genotype 2 or 3 No No No 12 wk with RBV with RBV if F3 or
in combination with Genotype 4 No or 24 wk with
RBV or in combina- 12 wk with RBV or No 12 wk with RBV RBV if F3 or cirrhosis
tion with PegIFN-α 24 wk with RBV if No or 24 wk with cirrhosis
No RBV if F3 or 12 wk with RBV or
and RBV F3 or cirrhosis cirrhosis No 24 wk with RBV if
No
PegIFN-α, RBV and Genotype 5 or 6 12 wk with RBV or No F3 or cirrhosis
simeprevir Genotype 1 or 4 24 wk with RBV if No
12 wk with RBV 12 wk with RBV or
F3 or cirrhosis No or 24 wk with 24 wk with RBV if
RBV if F3 or
12 wk with RBV or cirrhosis F3 or cirrhosis
24 wk with RBV if 12 wk with RBV or
No 24 wk with RBV if
F3 or cirrhosis
12 wk with RBV F3 or cirrhosis
Genotype 1 No or 24 wk with
RBV if F3 or No
PegIFN-α, RBV and Genotype 2 or 3 No No No cirrhosis
daclatasvir Genotype 4 No No No 12 wk with RBV or
No No No 24 wk with RBV if
Sofosbuvir and Genotype 5 or 6 12 wk with RBV or No No
simeprevir Genotype 1 or 4 24 wk with RBV if No F3 or cirrhosis
F3 or cirrhosis No
12 wk with RBV or 12 wk with RBV or
24 wk with RBV if 24 wk with RBV if
F3 or cirrhosis F3 or cirrhosis
12 wk with RBV or
24 wk with RBV if
F3 or cirrhosis
(continued on next page)
24