Page 34 - EASL POSTGRADUATE COURSE
P. 34
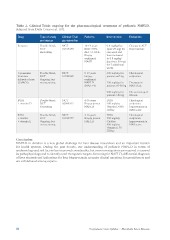
Table 2. Clinical Trials ongoing for the pharmacological treatment of pediatric NAFLD.
Adapted from Della Corte et al. [10].
Drug Type of study Clinical-Trial. Patients Intervention Endpoints
and status gov identifier
Losartan 12-19 years 0.4 mg/kg/day Change in ALT
Double blind, NCT BMI >85% (max 25 mg) for from baseline
RCT 01913470 ALT ≥3 UNL one week and
Recruiting Biopsy then increased Histological
NCT confirmed to 0.8 mg/kg/ endpoints:
Cysteamine Double blind, 01529268 NASH day (max 50 mg)
bitartrate RCT for 7 additional
delayed-release Ongoing, but NCT 8-17 years weeks
(CyNCh) not recruiting 02098317 biopsy-
confirmed 600 mg/day for
NCT NAFLD patients ≤65 kg
01934777 (NAS >4)
750 mg/day for Decrease in
4-16 years patients 65-80 kg NAS of ≥2
Biopsy proven
DHA Double blind, NAFLD 900 mg/day for No worsening of
+ vitamin D RCT patients >80 kg fibrosis
Recruiting 4-16 years
DHA Biopsy proven DHA Histological
+ choline Double blind, NAFLD 500 mg/day endpoints:
+ vitamin E RCT Vitamin D 800 Improvement in
Ongoing, but IU/day NAS score
not recruiting
DHA Histological
500 mg/day endpoints:
Choline Improvement in
400 mg/day NAS score
Vitamin E 78
IU/day
Conclusion
NAFLD in children is a new global challenge for liver disease researchers and an important burden
for health systems. During the past decade, our understanding of pediatric NAFLD in terms of
epidemiology and risk factors has improved considerably, but more investigations are required to unravel
its pathophysiology and to identify novel therapeutic targets. Screening for NAFLD, differential diagnosis
of liver steatosis and indications for liver biopsy remain as major clinical questions for practitioners and
are still debated among experts.
34 Postgraduate Course Syllabus • Metabolic Liver Disease
Adapted from Della Corte et al. [10].
Drug Type of study Clinical-Trial. Patients Intervention Endpoints
and status gov identifier
Losartan 12-19 years 0.4 mg/kg/day Change in ALT
Double blind, NCT BMI >85% (max 25 mg) for from baseline
RCT 01913470 ALT ≥3 UNL one week and
Recruiting Biopsy then increased Histological
NCT confirmed to 0.8 mg/kg/ endpoints:
Cysteamine Double blind, 01529268 NASH day (max 50 mg)
bitartrate RCT for 7 additional
delayed-release Ongoing, but NCT 8-17 years weeks
(CyNCh) not recruiting 02098317 biopsy-
confirmed 600 mg/day for
NCT NAFLD patients ≤65 kg
01934777 (NAS >4)
750 mg/day for Decrease in
4-16 years patients 65-80 kg NAS of ≥2
Biopsy proven
DHA Double blind, NAFLD 900 mg/day for No worsening of
+ vitamin D RCT patients >80 kg fibrosis
Recruiting 4-16 years
DHA Biopsy proven DHA Histological
+ choline Double blind, NAFLD 500 mg/day endpoints:
+ vitamin E RCT Vitamin D 800 Improvement in
Ongoing, but IU/day NAS score
not recruiting
DHA Histological
500 mg/day endpoints:
Choline Improvement in
400 mg/day NAS score
Vitamin E 78
IU/day
Conclusion
NAFLD in children is a new global challenge for liver disease researchers and an important burden
for health systems. During the past decade, our understanding of pediatric NAFLD in terms of
epidemiology and risk factors has improved considerably, but more investigations are required to unravel
its pathophysiology and to identify novel therapeutic targets. Screening for NAFLD, differential diagnosis
of liver steatosis and indications for liver biopsy remain as major clinical questions for practitioners and
are still debated among experts.
34 Postgraduate Course Syllabus • Metabolic Liver Disease