Page 18 - NON-INVASIVE TESTS FOR EVALUATION OF LIVER DISEASE SEVERITY AND PROGNOSIS
P. 18
HBV JOURNAL OF HEPATOLOGY
Prolonged treatment with antiviral therapy is associated with
resolution of liver fibrosis and regression of liver cirrhosis Recommendations
[269–271]. Non-invasive tests are an attractive strategy to moni-
tor changes in fibrosis. A significant decrease in LS and biomark- • Non-invasive assessment with either serum biomarkers
ers values, compared with baseline values, in HBV-infected or TE can be used to monitor improvement in liver
patients treated with analogs has been reported [272–281]. fibrosis during antiviral therapy. The correlation of
However, like for HCV patients, improvement of LS in HBV fibrosis improvement predicted by non-invasive
patients who start antiviral therapy when ALT is elevated may measurement with histology has yet to be determined
be related to normalization of ALT instead of fibrosis improve- (B2)
ment [274]. In this case, a LS measurement a few months after
commencing treatment and normalization of ALT is recom- • The impact of ALT normalization by antiviral therapy
mended as baseline to monitor the changes in liver fibrosis. The has to be considered on interpretation of the non-
value of non-invasive tests to predict the occurrence of com- invasive liver fibrosis assessment results (A1)
plications or survival in patients with undetectable HBV DNA
and cirrhosis prior to antiviral treatment remains to be deter-
mined [282–284].
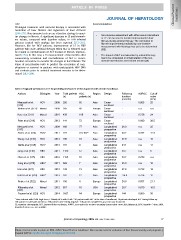
Table 8. Prognostic performance of TE for predicting development of HCC in patients with chronic liver disease.
Authors Etiologies Year Total patients HCC Region Design Follow-up AUROC Cut off
duration value
(n) patients (n) (months) (kPa)
Masuzaki et al. HCV 2008 265 85 Asia Cross- - 0.805 25
[312] sectional
Nahon et al. [313]^ Mixed 2009 265 66 Europe Cross- - n.a. n.a.
sectional
Kuo et al. [311] Mixed 2010 435 106 Asia Cross- - 0.736 24
sectional
Feier et al. [310] HCV 2013 144 72 Europe Cross- - 0.680 38.5
sectional
Masuzaki et al. HCV 2009 866 77 Asia Longitudinal 36.0 n.a. 25
[317] prospective
Akima et al. [314] HCV* 2011 157 41 (10)** Asia Longitudinal 40.7 0.787 12.5
prospective
Wang et al. [319] HCV 2013 198 10 Asia Longitudinal 47.8 n.a. 12
prospective
Narita et al. [318] HCV 2013 151 9 Asia Longitudinal 24.1 n.a. 14
prospective
Jung et al. [316] HBV 2011 1130 57 Asia Longitudinal 30.7 n.a. 8
prospective
Chon et al. [315] HBV 2012 1126 63 Asia Longitudinal 30.7 0.789 n.a.
prospective
Fung et al. [275] HBV 2011 528 7 Asia Longitudinal 35.0 n.a. 10
prospective
Kim et al. [321] HBV 2012 128 13 Asia Longitudinal 27.8 0.722 19
prospective
Kim Do et al. [320] HBV¥ 2013 162 12 Asia Longitudinal 24.0 0.736 12
prospective
Robic et al. [322] Mixed 2011 100 4 Europe Longitudinal 24.0 0.837 21.1
prospective
Klibansky et al. Mixed 2012 667 16 USA Longitudinal 28.7 0.870 10.5
[267]
prospective
Poynard et al. [323] HCV 2014 3927 84 Europe Longitudinal 144 0.860 50
prospective
^Liver cirrhosis with Child-Pugh class A. ⁄Mostly HCV with 85.4%. ⁄⁄41 patients with HCC at the time of enrollment, 10 patients developed HCC during follow-up.
All patients treated with interferon; àAll patients were HBeAg-negative; ¥All patients completed 2-year entecavir treatment.
TE, transient elastography; HCC, hepatocellular carcinoma; AUROC, area under the receiver operating characteristic curve; kPa, kilopascal; HCV, hepatitis C virus; HBV,
hepatitis B virus; n.a., not available.
Journal of Hepatology 2015 vol. xxx j xxx–xxx 17
Please cite this article in press as: EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J
Hepatol (2015), http://dx.doi.org/10.1016/j.jhep.2015.04.006
Prolonged treatment with antiviral therapy is associated with
resolution of liver fibrosis and regression of liver cirrhosis Recommendations
[269–271]. Non-invasive tests are an attractive strategy to moni-
tor changes in fibrosis. A significant decrease in LS and biomark- • Non-invasive assessment with either serum biomarkers
ers values, compared with baseline values, in HBV-infected or TE can be used to monitor improvement in liver
patients treated with analogs has been reported [272–281]. fibrosis during antiviral therapy. The correlation of
However, like for HCV patients, improvement of LS in HBV fibrosis improvement predicted by non-invasive
patients who start antiviral therapy when ALT is elevated may measurement with histology has yet to be determined
be related to normalization of ALT instead of fibrosis improve- (B2)
ment [274]. In this case, a LS measurement a few months after
commencing treatment and normalization of ALT is recom- • The impact of ALT normalization by antiviral therapy
mended as baseline to monitor the changes in liver fibrosis. The has to be considered on interpretation of the non-
value of non-invasive tests to predict the occurrence of com- invasive liver fibrosis assessment results (A1)
plications or survival in patients with undetectable HBV DNA
and cirrhosis prior to antiviral treatment remains to be deter-
mined [282–284].
Table 8. Prognostic performance of TE for predicting development of HCC in patients with chronic liver disease.
Authors Etiologies Year Total patients HCC Region Design Follow-up AUROC Cut off
duration value
(n) patients (n) (months) (kPa)
Masuzaki et al. HCV 2008 265 85 Asia Cross- - 0.805 25
[312] sectional
Nahon et al. [313]^ Mixed 2009 265 66 Europe Cross- - n.a. n.a.
sectional
Kuo et al. [311] Mixed 2010 435 106 Asia Cross- - 0.736 24
sectional
Feier et al. [310] HCV 2013 144 72 Europe Cross- - 0.680 38.5
sectional
Masuzaki et al. HCV 2009 866 77 Asia Longitudinal 36.0 n.a. 25
[317] prospective
Akima et al. [314] HCV* 2011 157 41 (10)** Asia Longitudinal 40.7 0.787 12.5
prospective
Wang et al. [319] HCV 2013 198 10 Asia Longitudinal 47.8 n.a. 12
prospective
Narita et al. [318] HCV 2013 151 9 Asia Longitudinal 24.1 n.a. 14
prospective
Jung et al. [316] HBV 2011 1130 57 Asia Longitudinal 30.7 n.a. 8
prospective
Chon et al. [315] HBV 2012 1126 63 Asia Longitudinal 30.7 0.789 n.a.
prospective
Fung et al. [275] HBV 2011 528 7 Asia Longitudinal 35.0 n.a. 10
prospective
Kim et al. [321] HBV 2012 128 13 Asia Longitudinal 27.8 0.722 19
prospective
Kim Do et al. [320] HBV¥ 2013 162 12 Asia Longitudinal 24.0 0.736 12
prospective
Robic et al. [322] Mixed 2011 100 4 Europe Longitudinal 24.0 0.837 21.1
prospective
Klibansky et al. Mixed 2012 667 16 USA Longitudinal 28.7 0.870 10.5
[267]
prospective
Poynard et al. [323] HCV 2014 3927 84 Europe Longitudinal 144 0.860 50
prospective
^Liver cirrhosis with Child-Pugh class A. ⁄Mostly HCV with 85.4%. ⁄⁄41 patients with HCC at the time of enrollment, 10 patients developed HCC during follow-up.
All patients treated with interferon; àAll patients were HBeAg-negative; ¥All patients completed 2-year entecavir treatment.
TE, transient elastography; HCC, hepatocellular carcinoma; AUROC, area under the receiver operating characteristic curve; kPa, kilopascal; HCV, hepatitis C virus; HBV,
hepatitis B virus; n.a., not available.
Journal of Hepatology 2015 vol. xxx j xxx–xxx 17
Please cite this article in press as: EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J
Hepatol (2015), http://dx.doi.org/10.1016/j.jhep.2015.04.006